
One sentence summary
In a study comparing osimertinib to platinum-based therapy plus pemetrexed in EGFR T790M–Positive Lung Cancer patients, osimertinib demonstrated significantly longer progression-free survival and greater efficacy, especially in those with advanced non–small-cell lung cancer that progressed after first-line EGFR-TKI therapy.
Background
Non-small-cell lung cancer (NSCLC) patients with EGFR-TKI sensitizing and T790M resistance mutations often face challenges in treatment due to the progression of the disease after first-line EGFR-TKI therapy. Osimertinib, a third-generation, irreversible EGFR-TKI, has shown promise in early-phase clinical trials for these patients. Meanwhile, platinum-based therapy plus pemetrexed remains a standard treatment option. This study aims to compare the efficacy of osimertinib with that of platinum-based therapy plus pemetrexed in this specific patient population.
Method
- In a phase 3, open-label, international trial, 419 patients with T790M-positive advanced non–small-cell lung cancer, who showed disease progression post first-line EGFR-TKI therapy, were randomized.
- Patients were assigned in a 2:1 ratio to receive either oral osimertinib (80 mg daily) or intravenous pemetrexed (500 mg/m^2) combined with carboplatin (AUC5) or cisplatin (75 mg/m^2) every 3 weeks for up to six cycles, with maintenance pemetrexed permitted.
- The primary measurement for the study’s success was the investigator-assessed progression-free survival.
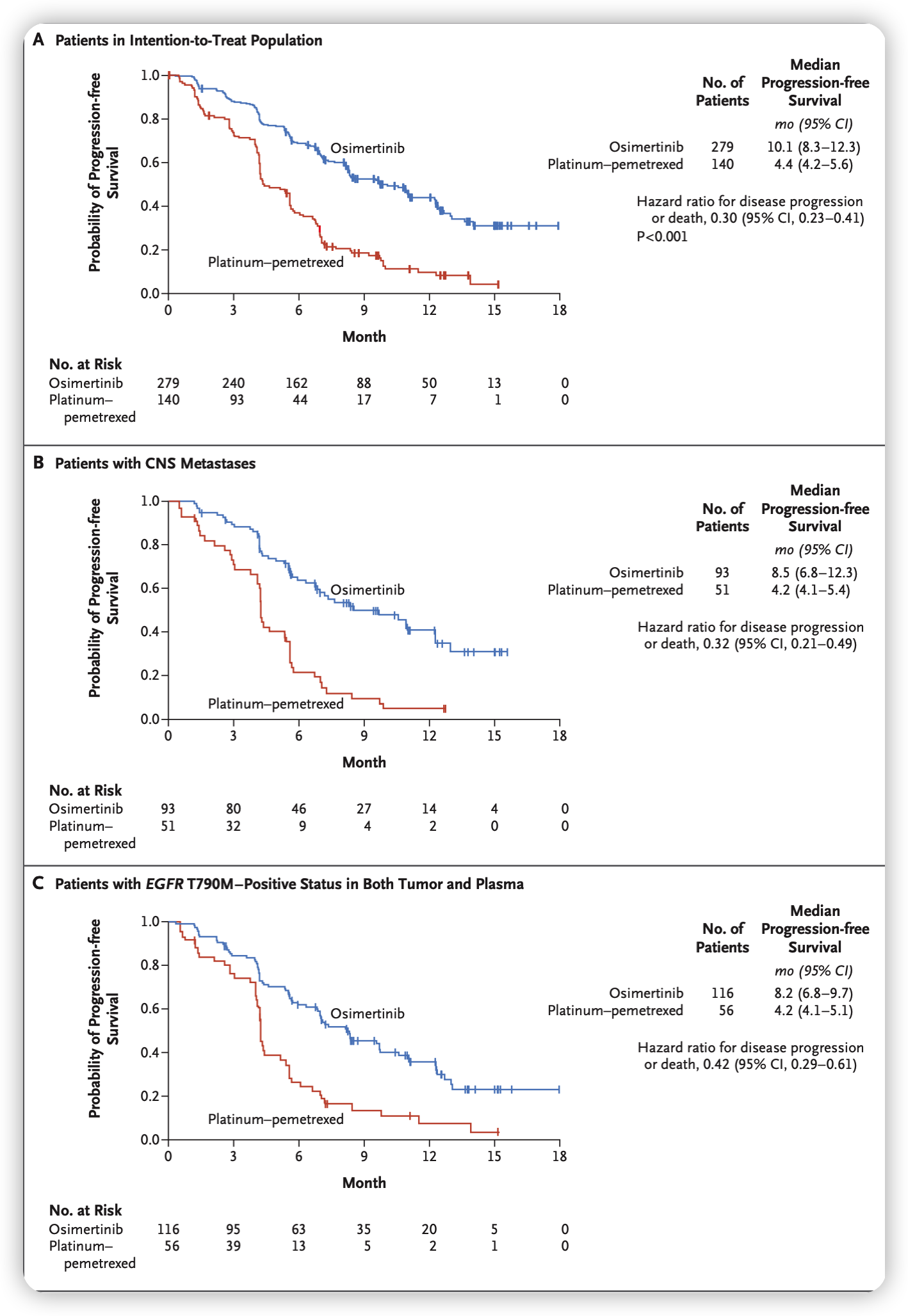
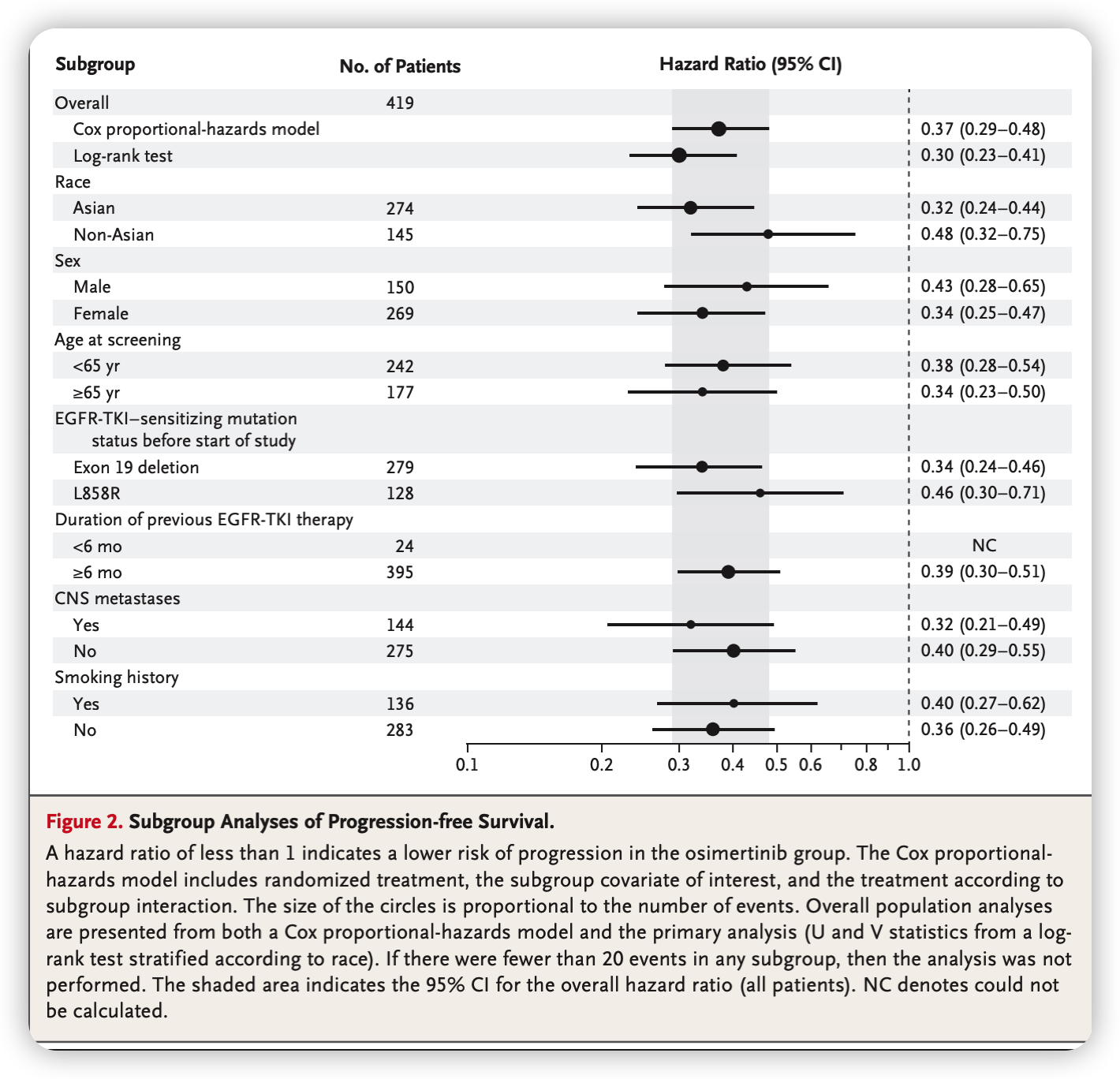
Discussion
- In the trial, patients with T790M-positive advanced NSCLC who received osimertinib showed better response rates compared to those receiving platinum therapy plus pemetrexed after first-line EGFR-TKI therapy.
- Osimertinib treatment resulted in a longer duration of progression-free survival for these patients.
- The progression-free survival benefit with osimertinib was observed across all predefined subgroups.
- These subgroups demonstrated hazard ratios of less than 0.50, indicating a significant reduction in the risk of disease progression or death with osimertinib treatment.
Reading summary
What is the motivation?
Despite the initial effectiveness of EGFR-TKIs as the standard first-line therapy for advanced non–small-cell lung cancer with a mutant EGFR, disease progression occurs in most patients after 9 to 13 months, with around 60% exhibiting the T790M mutation, which reduces the binding efficacy of first and second-generation EGFR-TKIs.
What is the novelty & contribution?
- Osimertinib, an oral EGFR-TKI, has been identified as selective for both EGFR and T790M resistance mutations and has shown activity in the central nervous system.
- Previous studies, including the phase 1 component of AURA and two subsequent phase 2 studies, have demonstrated promising results for osimertinib in treating T790M-positive NSCLC.
- The AURA3 trial, a phase 3 study, was designed to confirm the superiority of osimertinib over the standard platinum therapy plus pemetrexed in patients with T790M-positive advanced NSCLC post first-line EGFR-TKI therapy.
- The results from AURA3, as presented, provide evidence supporting osimertinib’s efficacy, making it a potential new standard of care for this patient group.