One sentence summary
Research indicates that SARS-CoV-1 survivors immunized with the BNT162b2 mRNA vaccine produce potent antibodies capable of neutralizing current and potential future SARS-CoV-2 variants, suggesting the possibility of a universal sarbecovirus vaccine.
Background
The Covid-19 pandemic, initiated by SARS-CoV-2 in December 2019, shares genetic similarities with SARS-CoV-1, the virus responsible for the 2002–2003 SARS outbreak which resulted in over 8,000 infections and over 700 deaths globally. Both SARS-CoV-1 and SARS-CoV-2 belong to the SARS-related coronavirus species, with distinct phylogenetic classifications. Despite their genetic differences, the urgent need for effective vaccines against these and potential future strains is evident.
Method
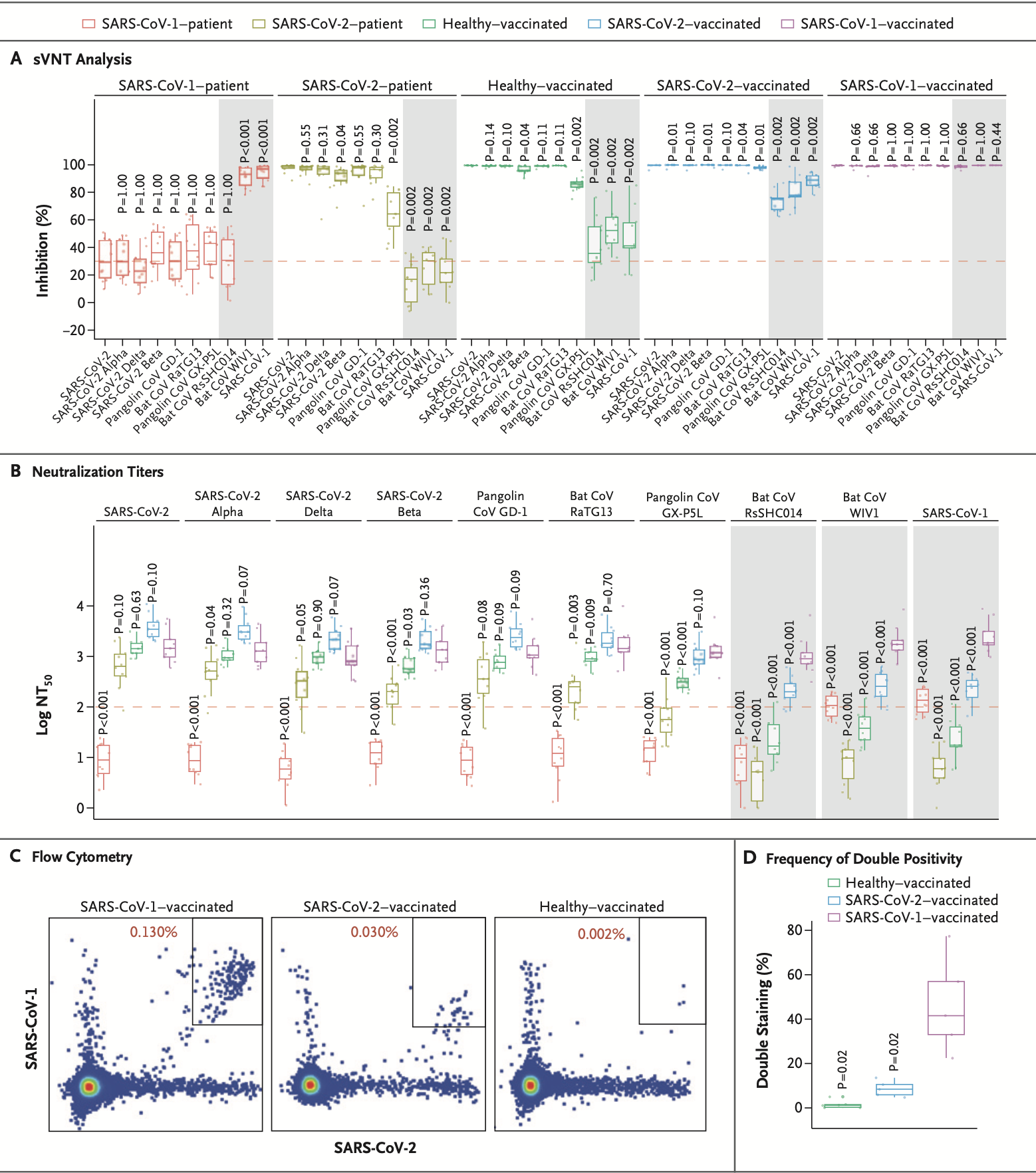
- Two surrogate virus neutralization tests (sVNTs) were employed, with the singleplex sVNTs for SARS-CoV-1 and SARS-CoV-2 previously described and the SARS-CoV-2 sVNT kit commercialized as cPass.
- For multiplex sVNTs, the Luminex platform was adapted, using AviTag-biotinylated receptor-binding domain (RBD) proteins from 10 different sarbecoviruses coated on microspheres, which were then preincubated with serum and subsequently with phycoerythrin (PE)–conjugated human angiotensin-converting enzyme 2 (ACE2).
- B-cell profiling was conducted using flow-cytometry analysis on cryopreserved peripheral blood mononuclear cells.
Discussion
- The emergence of SARS-CoV-2 variants underscores the need for a vaccine that can address both current and future variants, leading to the concept of a pan-sarbecovirus vaccine.
- The study’s findings suggest that potent cross-clade pan-sarbecovirus neutralizing antibodies can be induced, offering broad-spectrum protection against known and potential future sarbecoviruses.
- The presence of these antibodies, especially in SARS-CoV-1 survivors who have been immunized, paves the way for the feasibility of a comprehensive pan-sarbecovirus vaccine strategy.
Reading summary
What is the motivation?
- The motivation behind this study is the global challenge posed by the emergence of SARS-CoV-2 variants, which may compromise the effectiveness of current vaccines. Recognizing the potential threat of both existing and future variants, as well as other sarbecoviruses that might emerge, there’s a pressing need to develop a vaccine that offers broader protection. The ideal solution would be a pan-sarbecovirus vaccine that not only addresses known variants but also provides immunity against potential future strains, ensuring better preparedness for subsequent outbreaks and safeguarding global health.
What is the novelty & contribution?
- The novelty of this study lies in its exploration of the potential for a pan-sarbecovirus vaccine, a concept that goes beyond the current generation of vaccines targeting specific SARS-CoV-2 strains. By examining the neutralizing antibody responses in survivors of SARS-CoV-1 infection who have been immunized with the BNT162b2 mRNA vaccine, the study provides groundbreaking data on the induction of potent cross-clade pan-sarbecovirus neutralizing antibodies. These antibodies are not only effective against known SARS-CoV-2 variants but also against sarbecoviruses found in bats and pangolins, which have the potential to infect humans.
- The study’s contribution is significant as it paves the way for the development of a more comprehensive vaccine strategy, offering broader protection against a wider range of coronaviruses, both known and yet to emerge.